The Repair of Reinforced Concrete
John Broomfield
Recent developments in the repair of reinforced concrete include modern electrochemical techniques that can minimise the interference with the structure, an important factor in building restoration.
 |
|
| The Hoover Building, Perivale, London (built 1932-35): a spectacular example of reinforced concrete construction, now occupied by Tesco |
We generally think of concrete as a modern building material, yet it is one of the oldest and most durable building materials. Its earliest known use was for a hut floor in former Yugoslavia, dating from 5600BC: later, more notable examples included the Great Pyramid at Giza and the Parthenon in Rome.
Although the Romans experimented with bronze reinforcement, reinforced concrete as we know it today dates from the mid 19th century following the introduction of Portland cement concrete in 1854 when it was patented by Joseph Aspeden in Wakefield. Steel reinforced boats and plant tubs were made in the 1850s, and a patent was taken out in 1854 by William Wilkinson for a method of building fireproof buildings using strips of iron embedded in mass concrete. Wilkinson showed that he understood where tension steel was needed in his flat ceilings, where wire ropes were embedded following the line of tension in the upper parts of beams over supports and in the lower parts in the mid-span.
Steel has the advantage of having the tensile strength that concrete lacks, and is highly compatible in its chemical and physical characteristics as we will see later. The matching of thermal expansion coefficients is critical to the versatility of reinforced concrete.
DETERIORATION MECHANISMS
Like masonry and brick, reinforced concrete structures deteriorate under attack from external elements such as freeze-thaw damage (the expansion of frozen moisture within the structure as it thaws), and erosion. In a composite man-made material such as concrete there are additional mechanisms caused by the greater complexity of its composition. Of particular concern today is the alkali silica reaction in the concrete and the corrosion of the reinforcing steel, both of which are affected by the alkalinity of Portland cement concrete. Portland cement is made by burning constituents which include lime in a kiln and grinding the result to a fine powder. This produces a highly alkaline material which reacts with water and hardens. When it is added to coarse and fine aggregate and mixed with water, the cement combines with the aggregate and hardens to form concrete. The hardening process (hydration reaction) is complex and continues over many months if not years, depending on the amount of water in the mix. There must be excess water for workability and a pore network therefore develops as it dries out. Excess calcium hydroxide and other alkaline hydroxides are present in the pores and a solution of pH 12.0 to 14.0 develops (pH 7.0 is neutral; values below indicate acidity, and alkalinity above). It is this pore network and the solutions it contains that are critical to the durability of the concrete.
ALKALI SILICA REACTIVITY (ASR)
ASR occurs if the wrong aggregates are used in the mix. Some silicaceous minerals, including quartzes and opals, react with water in a high alkaline environment to form silica gel, a material used to absorb moisture. As silica gel swells when it absorbs moisture, the material can cause concrete to crack, and white, weeping deposits of silica appear. In many cases ASR is superficial and harmless, but it is unattractive and difficult to treat. The most effective remedy is to dry out the structure.
Many if not most types of concrete incorporate some material which is susceptible to ASR. However very few structures show signs of significant ASR damage, as the reactive aggregate components which cause the problem are consumed in the process. Those areas of the United Kingdom where ASR is prevalent are now well known and the quarries responsible have been identified.
CORROSION OF REINFORCING STEEL
Although the alkalinity within the concrete pore structure can lead to ASR, the high pH value also provides a protective coating of oxides and hydroxides on the surface of the steel reinforcement. Without this layer, which is known as a 'passive' film, the steel would be exposed to the air and moisture in the pores, leading to rapid corrosion. It is the main chemical reason why reinforced concrete is a durable construction material. The layer is durable and self repairing, and it can last for hundreds of years if the alkalinity is maintained. However, the passive layer itself can be attacked by chlorides in salt and the alkalinity of the concrete can be reduced by reaction with atmospheric carbon dioxide, a process known as 'carbonation'.
DETERIORATION THROUGH CARBONATION
Carbon dioxide, which is present in the air in proportions of around 0.3 per cent by volume, dissolves in water to form a mildly acidic solution. Unlike other acids that may chemically attack and etch the surface of the concrete, this acid forms within the pores of the concrete itself where the carbon dioxide dissolves in any moisture present. Here it reacts with the alkaline calcium hydroxide forming insoluble calcium carbonate. The pH value then drops from more than 12.5 to about 8.5. The carbonation process moves as a front through the concrete, with a pH drop across the front. When it reaches the reinforcing steel, the passive layer decays when the pH value drops below 10.5. The steel is then exposed to moisture and oxygen and is susceptible to corrosion.
Concrete inside the building frequently carbonates totally without any sign of deterioration as the concrete dries out, leaving the steel exposed to air but not moisture. Problems are seen externally where concrete is exposed to the elements and in certain situations internally, such as kitchens and bathrooms, where the concrete is susceptible to condensation or water-leakage. External facades are particularly vulnerable, especially where cladding panels have poorly placed handling steel that is near the surface. Carbonation does not have to penetrate far and the concrete quality may be of poor quality.
DETERIORATION DUE TO CHLORIDE
Salt causes corrosion by a different mechanism. When dissolved in water sodium chloride forms a versatile, highly corrosive solution of sodium ions (Na+) and chloride ions (Cl-). Salt is used for de-icing roads and its presence in sea water is a major problem for reinforced concrete structures. The very mobile chloride ions disperse through concrete pores in solution and where they come into contact with the reinforcing steel they attack the passive layer. Steel oxidises in the presence of air and water to form rust which has a volume of up to 10 times that of the steel consumed. As concrete has a low tensile strength it will crack when as little as a tenth of a millimetre of steel has been consumed. Horizontal cracks form, causing corners to 'spall' and surfaces to 'delaminate' as the reinforcement's concrete cover becomes detached and falls away in sheets. The consequence can be seen on the underside of road bridges and many buildings and structures beside the sea.
|
THE CORROSION MECHANISM
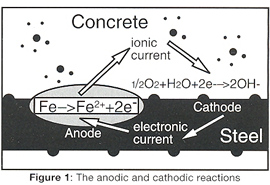
Corrosion of steel reinforcement occurs by an electrochemical process which involves exchanges of electrons similar to that which occurs in a battery. The important part of the mechanism is the separation of negatively charged areas of metal or 'anodes' where corrosion occurs and positively charged areas or 'cathodes' where a harmless charge balancing reaction occurs (Figure 1). At the anode the iron dissolves and then reacts to form the solid corrosion product, rust. The rust is formed at the metal/oxide interface, forcing previously formed oxide away from the steel and compressing the concrete, causing it to spall.
REPAIR TECHNIQUES
If corrosion of steel in concrete is suspected, a deterioration survey must be carried out to identify the cause, mechanism and extent of corrosion. An inadequate investigation can lead to higher costs and inadequate repairs. There are certain tests which are specific to the corrosion assessment of steel in concrete, relying on the electrochemical nature of the corrosion process. These are half-cell potential measurement, resistivity measurement and corrosion rate measurement. Further examination of these techniques is beyond the scope of this article, and a reading list is provided below for further reference.
PHYSICAL
The obvious thing to do when confronted with corrosion damage is to cut out the damaged areas, replace any steel weakened by section loss and put back good quality concrete. However there are several problems with this approach:
- cutting out the area of damage may leave many areas that are about to crack and spall
- as a result of the electrochemical nature of the corrosion process, repairs can actually lead to an acceleration of corrosion in adjacent areas, especially with chloride-induced corrosion, as the removal of the corroding anode also cause the loss of the protective cathodes around it and new anodes form when the material is renewed
- the repairs may be visually intrusive as it is very difficult to match the concrete used for repair to the colour and texture of the original, and it is almost impossible to get the new material to weather in the same way
- extensive concrete removal requires substantial temporary support, adding to the complexity of the project as well as expense
- coatings and barriers can be very effective if the amount of chloride at the depth of the reinforcement is below the chloride threshold or if the depth of carbonation is less than the cover depth. Penetrating sealers such as siloxy silanes have been shown to help to dry out concrete if leaks are repaired and the amount of direct water on the concrete is reduced. These are colourless and penetrate the surface leaving the appearance unaffected.
However, silanes are not suitable for carbonated concrete. Anti-carbonation coatings must be crack-bridging surface coatings to keep out carbon dioxide. However, coatings membranes and sealers are all useless if corrosion has already begun and direct water impingement is not minimised. Coatings, penetrating coatings and barriers can also be effective in slowing or stopping ASR by drying out the concrete.
 |
||
 |
ELECTROCHEMICAL
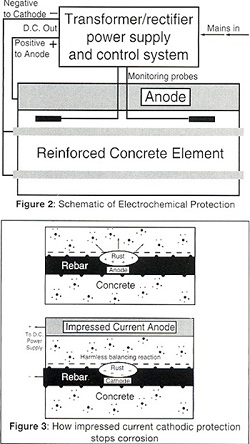
The movement of charged ions and the separation of anodes and cathodes along the steel creates some of the problems but also offers us some solutions to the corrosion of steel in concrete, as corrosion can be stopped by making all the steel a cathode (Figure 1). This is done by putting an external anode on the surface or embedding it in the concrete (Figure 2). The DC power supply, known as a transformer rectifier, will then pass current between the anode and the reinforcing steel.
This electrochemical rehabilitation approach can be used in three different ways: cathodic protection; electrochemical chloride migration or 'desalination'; and re-alkalisation.
CATHODIC PROTECTION (CP)
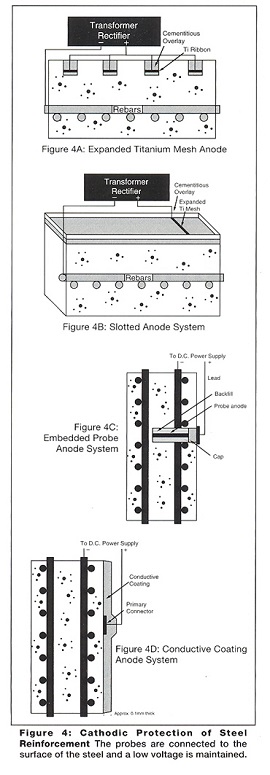
In this process the anodes, power supply and control systems are permanent, and a range of anodes can be used (Figures 3 and 4). The aggressive anodic reaction is isolated to a corrosion resistant anode while the harmless cathodic reaction occurs at the surface of the steel reinforcement. This process creates additional hydroxyl ions, rebuilds the passive alkaline layer and repels chloride ions.
CP has been used on hundreds of reinforced concrete structures around the world and has potential for the conservation of historic brick and stone masonry, terracotta and statuary where steel and iron has been used to provide reinforcement or a structural frame.
ELECTROCHEMICAL CHLORIDE MIGRATION (DESALINATION)
This process uses a temporary anode, power supply and monitoring system to apply 50 volts direct current to the steel. The positive charge repels the negatively charged chloride ions and rebuilds the passive layer over a period of four to six weeks. Although less well proven than CP, the technique has been used to successfully treat more than 50 structures in the UK, continental Europe and North America.
RE-ALKALISATION
This system is the equivalent of desalination for carbonated structures. It relies on the principle that the hydroxyl ions produced at the cathode re-alkalise the concrete from the reinforcement outwards. This is linked with a wet anode at the surface that contains calcium carbonate, which moves under electro-osmotic pressure and re-alkalises the concrete from the surface inwards.
There are more than one hundred re-alkalisation projects completed in the UK and on the continent. One of the earliest was the renovation of the Hoover Factory beside the M40 at Perivale, NW London (see illustration at top of page).
These specialist treatments require expert advice to check that the structure is suitable and that the best system is applied. There must be steel continuity, separation between steel and anodes and reasonable concrete quality before these techniques can be considered as cost effective and technically sound for a particular structure.
CORROSION INHIBITOR REPAIR TECHNIQUES
A recent development is the impregnation with chemical corrosion inhibitors which are widely used in the power generation, chemical and manufacturing industries. Recently, attempts have been made to introduce these chemicals into hardened concrete. If successful, then these could be good, relatively simple methods of increasing the life span, reducing maintenance and providing a 'minimum intervention' method of slowing or stopping corrosion.
SUMMARY
Corrosion of steel in concrete can be seen to be a significant problem for many reinforced concrete structures if moisture is present. If there is no salt to cause corrosion in the short term, carbonation will affect most structures over the centuries. If the structure cannot be kept dry then there is a range of techniques that can be used depending on the structure, its condition and the cause and extent of the problem.
Electrochemical techniques can reduce the amount and extent of patch repairs, and leave the appearance unchanged with probes embedded in the concrete or a surface coating, depending on requirements and conditions. Chemical impregnation with corrosion inhibitors is also under investigation as a further option.
Alkali silica reaction is a chemical attack of the aggregates in the presence of the alkalinity of the concrete and moisture. If the concrete can be kept dry then ASR will be minimised. Most ASR damage is unsightly rather than structurally dangerous.
Recommended Reading
- JP Broomfield, 'Assessing Corrosion Damage on Reinforced Concrete Structures' in Corrosion and Corrosion Protection of Steel in Concrete, Edited by R Narayan Swamy, Sheffield Academic Press, 1994
- Cathodic Protection of Reinforced Concrete - Status Report, Report No. SCPRC/001.95, Society for the Cathodic Protection of Reinforced Concrete, London, 1995
- CC Stanley, Highlights in the History of Concrete, British Cement Association, Crowthorne, Berks, 1986
Concrete Society Technical Reports:
- No 26 Repair of Concrete Damaged by Reinforcement Corrosion,
1994
- No 36 Cathodic Protection of Reinforced Concrete, 1989