Hydraulicity Revisited
Edward Walker
 |
| Prompt natural cement being used for the repair of Portreath’s harbour wall: this binder is less dense and more porous than modern Portland cements and closer to the early Portland cements used in the late 19th century. (Photo: Cornish Lime Company) |
In recent years the use of NHL mortars has been called into question following new research and the popularity of hot-mixed mortars. This has shed light on properties of the binders used which many specifiers found surprising, and some are understandably concerned that we may have been inadvertently specifying repairs that were inappropriate or less appropriate than expected. We are now in a much better position to re-evaluate the benefits and the limitations of these materials scientifically, to ensure that all masonry repairs are made using the most appropriate technologies.
The purpose of this article is to provide a simplified account of the materials and the often complicated chemical reactions involved. This story has been confused by centuries of differing terminology and vernacular references. The following summary covers some of the technical elements, hopefully broken down into manageable chunks, but some technical terminology and chemical notation is unavoidable. Providing a complete picture would require volumes, and even Boynton’s Chemistry and Technology of Lime and Limestone does not cover the ‘hydraulicity’ of this discussion, despite being perhaps the most definitive guide to lime production ever written.
TERMINOLOGY |
LIME CALCINED STONE HYDRAULICITY NATURAL HYDRAULIC LIME (NHL) POZZOLAN CEMENT |
MORTAR AND ITS COMPONENTS
A binder is the component of a mortar which allows stiffening or binding of separate components to create a hardened composite. Limestone processed in a kiln has been the predominant source of binders for mortars and concrete for at least 5,000 years and it remains the backbone of construction today. Whether it is an air lime, a hydraulic lime, a natural cement or a modern Portland cement, it all starts with limestone of some description being chemically transformed in a hot kiln.
Non-hydraulic limes typically contain lime and unburnt stone as well as small proportions of the hydraulic compounds C2S/C3S, as outlined in the table above. This is because some silica is always present in limestones as none is ever truly pure.
Most hydraulic components interact with one another during hydration and small changes in trace elements can have significant impacts on final hydration products and crystallography.
The main constituents of lime (including hydraulic limes) after calcination are shown in the table above. The proportions may vary, but natural hydraulic limes will usually contain everything in this table. However, most of the commercially available limes in Europe at the moment contain insignificant quantities of C3S, C3A and C4AF. The strength of the lime predominantly depends on the ratio between belite (C2S) and calcium hydroxide along with both the particle size and the amount of water required to make a workable mortar.
Natural cements are essentially NHLs with very little or no calcium hydroxide remaining; the binders are predominantly belite.
WORKS REQUIRING CONSENT |
|
UNBURNT STONE |
Mostly limestone or silica, raw stone which hasn’t reacted or calcined in the kiln |
REACTED LIME |
Calcium oxide: on mixing with water this should slake over time to produce calcium hydroxide |
DICALCIUM SILICATE |
Also known as C2S or Belite, this is the main hydraulic component in natural hydraulic limes (NHLs) and natural cements |
TRICALCIUM SILICATE |
Also known as C3S or Alite, this is the main hydraulic component in Portland cements |
TRICALCIUM ALUMINATE |
Also known as C3A, this is typically considered undesirable in hydraulic binders as it can lead to flash setting, high heat generation after initial hydration and sulphate attack |
TETRACALCIUM ALUMINOFERRITE |
Also known as C4AF or Brownmillerite, has very little effect on binder performance by itself, but can give a weak early set and helps accelerate other parts of the binder |
Ordinary Portland cements (OPCs) have changed over many years and are now predominantly alitic (C3S) binders with a proportion of belite. Historic OPCs burnt in vertical shaft kilns had different crystal structures to modern OPCs and higher belite contents, causing historic cements to be dramatically weaker. Consequently, they created significantly less dense mortars than their modern counterparts. To put this in perspective; modern Portland cements can have a density between 1.4–2Kg/L, whereas historic Portland cements would have been closer to modern natural cements, like VICAT’s Prompt which has a density of 1Kg/L. Density differences influence the weight of binder per cubic metre as mortars are mixed by volume.
REACTIONS, SETTING AND CURING
Quicklime is calcium oxide; the most commonly available in the UK is CL90 calcium oxide – the 90 refers to the fact that it has a calcium oxide content of 90 per cent or higher. When water is added the quicklime slakes, releasing a large amount of heat and forming a CL90 calcium hydroxide.
‘Air lime’ is the term commonly used to describe a CL90 calcium hydroxide, but the material is also known as ‘putty lime’, ‘non-hydraulic lime’ and ‘fat lime’. When used in a mortar this binder stiffens after placement through loss of water, it does not ‘set’ or ‘cure’ and only hardens by carbonation over time. In this process carbon dioxide is absorbed from the atmosphere, and the lime reacts with it in the presence of water to form calcium carbonate. This will not occur without air or water, and deep within a wall it can take years to react.
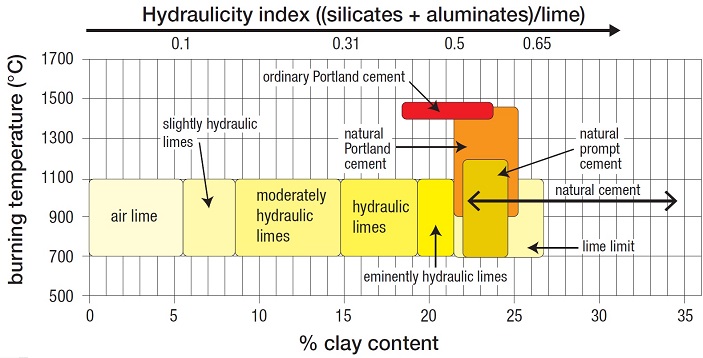 |
| The table shows hydraulicity increasing with increasing clay (and/or silica) content, from air limes on the left (up to 5%) to eminently hydraulic limes and cements on the right. The ‘lime limit’ is where any increase in the proportion of silica/clay begins to have little or no effect on hydraulicity because there is no more lime to combine: everything to the right of this is therefore a natural cement. (Table reproduced by kind permission of the Louis Vicat Foundation) |
Alite and belite on the other hand, form a chemical set on addition of water and must be damp-cured to develop strength. Both are varied in chemistry due to the minor components found in the source rocks, as well as calcining temperature and residence time in the kiln, but belite is typically simplified to di-calcium silicate, which reacts as per the following simplified equation;
2 (CaO)2·SiO2 + 8.6 H2O ![]() 3.4 (CaO)·2(SiO2)2·8(H2O) + 0.6 Ca(OH)2
3.4 (CaO)·2(SiO2)2·8(H2O) + 0.6 Ca(OH)2
Belite + water ![]() calcium silicate hydrate + calcium hydroxide
calcium silicate hydrate + calcium hydroxide
Alite is typically simplified to tri-calcium silicate which reacts as per the following simplified equation;
2 (CaO)3·SiO2 + 10.6 H2O ![]() 3.4(CaO)·2(SiO2)·8(H2O) + 2.6 Ca(OH)2
3.4(CaO)·2(SiO2)·8(H2O) + 2.6 Ca(OH)2
Alite + water ![]() calcium silicate hydrate + calcium hydroxide
calcium silicate hydrate + calcium hydroxide
Both belite and alite form the same resultant compounds; calcium silicate hydrate and calcium hydroxide. If you compare the equations you can see alite produces more calcium hydroxide than belite. When adjusting for density, alite produces approximately 3.2x the calcium hydroxide produced by belite.
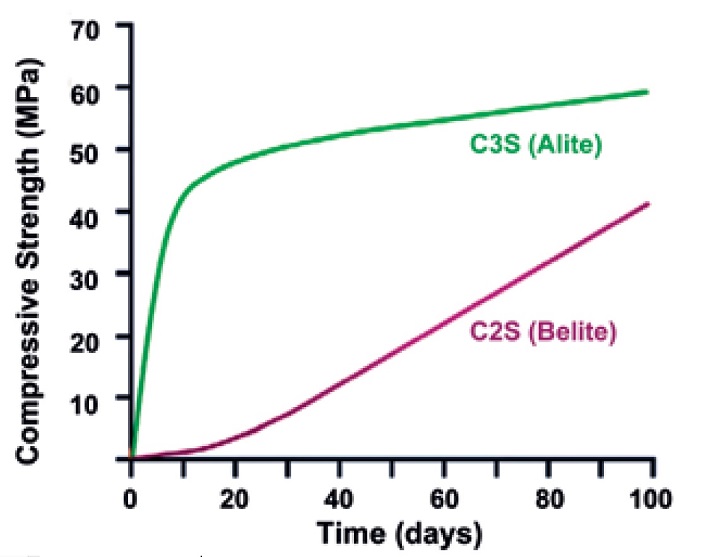 |
| Compressive strength development in pastes of pure cement compounds over 100 days (Table: Sydney Mindess et al 2003) |
Over time calcium silicate hydrate, the predominant compound from alite and belite reactions, will carbonate to form calcium carbonate and silica gel. This means that samples which were hydraulic limes used hundreds of years ago may now be interpreted as non-hydraulic or feebly hydraulic mortars through analysis.
COMPARING BINDER COMPONENTS
Air lime mortars are the lowest density and the weakest, and will give the highest vapour permeability – often desirable characteristics when used in the repair of a fragile historic masonry. Their hardened structure is relatively independent from water/binder ratio as they first stiffen through loss of water before slowly carbonating. Binder/aggregate ratio, aggregate properties and compaction dictate mortar performance:
• higher binder contents giving a more porous material
• limestone aggregates have been shown to increase both porosity and physical strength
• compacting the mortar as it begins to stiffen increases the density and final strength once carbonated, and will also enhance other final mortar performance metrics.
On the downside, air lime mortars need more water than other binders, which will increase plastic shrinkage through water loss. However, if plastic shrinkage is managed through tempering or reworking, larger visible cracks shouldn’t occur. Air lime mortars should be kept slightly damp in their infancy to promote carbonation as both water and carbon dioxide is required as part of the chemical reaction, but not too wet as the water can impede gas diffusion, slowing carbonation.
 |
 |
| Left; high level repairs are invariably exposed to a greater degree of attrition from wind and rain and benefit from a hydraulic set, as here at St Michael’s Mount. Right; Huer’s Hut, a lookout above Newquay, Cornwall was finished with a lime shelter coat. (Both photos: Cornish Lime Company) | |
Mortars made with binders which are primarily blends of lime, belite or alite have a hardened structure which is dependent on the water/binder ratio when the material sets. Binder/aggregate ratio and aggregate properties also have an effect; a higher binder content increases the strength and decreases the porosity, the level of decrease is dependent on the belite/alite ratio with higher alite contents being much less permeable. The aggregate minerology and grading can have varying impacts on strength, water/binder ratio and shrinkage.
Belite takes a long time to develop strength; two to three years is a realistic timescale to achieve close to the final strength. Belitic mortars should be kept damp in the early stages to ensure full hydration of the hydraulic components and to aid in carbonation of any lime content.
Alite develops strength quite quickly, it’s typically accepted that 28 days of damp curing is around 90 per cent of the final material strength. Alitic mortars should be kept damp in the early stages to promote full hydration of the hydraulic components.
CLASSIFICATION OF LIMES UNDER EN459
EN459 is a manufacturing standard designed to ensure that production is consistent and reliable. Complying with the standard permits the CE marking of products and allows manufacturers to sell these binders within the EU economic area.
 |
| A severe marine environment would pose particular problems for an air lime mortar. For the repair and consolidation of these chimney stacks on a scheduled monument on Lundy Island, a hydraulic set was essential. (Photo: Old Light Building Conservation) |
There is a common misunderstanding amongst many who use limes that NHL designations are the given strength for a binder and are indicative of real-life mortar performance. The number classification assigned to NHLs is only indicative of their strength when mixed at approximately 1:1.3, with an engineered, unrealistic sand and with a water/binder ratio which is unworkable. The mortar is then mechanically dropped to remove air from the samples and then tested at 28 days. This classification permits manufacturers to ensure their product is consistent with the standard but nothing else; diligent manufacturers recognise this and supply data to show longer-term strengths when used with realistic sands, mix ratios and workabilities.
COMMON CONCERNS
A growing number of people are concerned about how limes are classified as the test data has no relevance to real practical application. Some of these concerns are valid and some not, below we will examine some of the commonly highlighted issues.
There is a permitted overlap between NHL binders in EN459; this means that one binder could be sold as all three strengths.
While this is technically true, it is very unlikely to actually happen. NHLs are made from a naturally occurring material, so they can vary in strength when taken from different parts of the quarry and tested in the method specified in EN459. Quality assurance data is used to form a bell curve which is overlaid onto the EN459 banding structure; this bell curve will broadly fall into one of the categories. To ensure the standard is followed and to ensure test compliance, all NHL binder manufacturers are externally audited yearly and are subject to unannounced spot checks throughout the year.
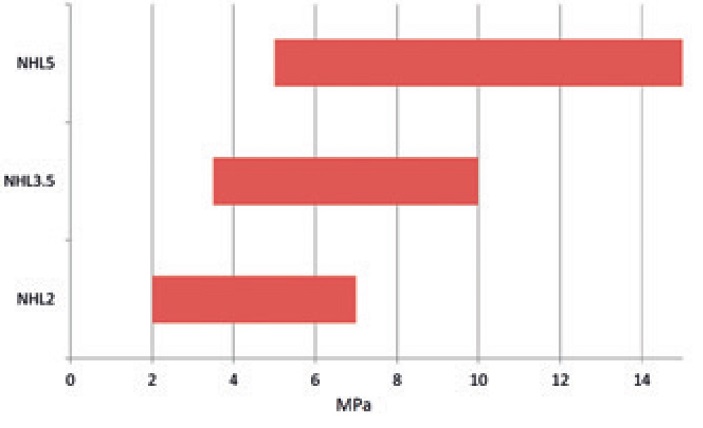 |
| NHL strengths as classified by EN459 |
Lime binders and mortars are tested at 28 days; this is nowhere near completely cured/carbonated and give unrealistic results.
This is correct; the current standards for both lime binders (EN459) and mortars/renders (EN998) require testing at 28 days even though they are not fully reacted. What must be realised is that both EN459 and EN998 are manufacturing standards and are only relevant for compliance of manufacturing; they are not designed for specification purposes. A 28 day period is reasonable for manufacturing compliance, but requiring test data to be produced for six or 24 months would be impractical and, from a quality-assurance perspective, meaningless: the aim of the standards are to prove the product is consistent and not that it performs in a certain way.
NHLs keep reacting over time; they increase in density and decrease in porosity.
This is also true; however, they still maintain an open pore structure. To elaborate on this we must refer back to the previous section about reactions, setting and curing; belitic binders release some lime while they hydrate and cure, this lines the pore structure of a mortar similar to the build-up of cholesterol in an artery. Where this differs from an alitic binder is that alite produces much more lime, meaning that rather than just lining the pore it blocks it, often overflowing out of the hardened binder matrix and exhibiting as lime bloom. This also partially explains why air lime mortars are the most porous; they don’t have any additional lime being deposited in the initial hydration phase.
If NHL mortars were impervious they wouldn’t be able to carbonate; at 20mm depth you can expect an NHL mortar to be completely carbonated within 1.5–2 years, a 20mm depth of modern Portland cement mortar would need around 400 years to fully carbonate.
 |
 |
| Left, a wall pointed with a mortar that was too hard and impervious for the masonry, forcing moisture to evaporate though the stones between: Right, free lime leaching from the core of a terracotta balustrade in Newcastle, which was probably caused by a breeze-concrete fill containing granulated blast furnace slag. (All photos this page: Jonathan Taylor) | |
Air lime mortars also decrease in porosity as they react, as there is approximately a 10 per cent volume increase in crystal size from calcium hydroxide to calcium carbonate. Furthermore the higher unslaked oxides available at time of placement in these mortars increases the risk of density change blocking pores and increasing strength through unintended densification.
Pozzolan mortars with air limes can be more porous than NHLs, so why not just use them?
Pozzolans are hugely varying in their reactivity, their workable behaviour and their local appropriateness. Pozzolans can impact every hardened mortar property, sometimes in ways which are undesirable in conservation. If you can find a good consistent source of pozzolan with repeatable results and performance then there is no reason not to use them, but make sure you understand how it affects the mortar before use.
Pozzolans can be more or less consistent than NHLs in performance, it entirely depends on the source and quality-assurance methodology of each; like every manufactured product – some manufacturers and sources are better than others.
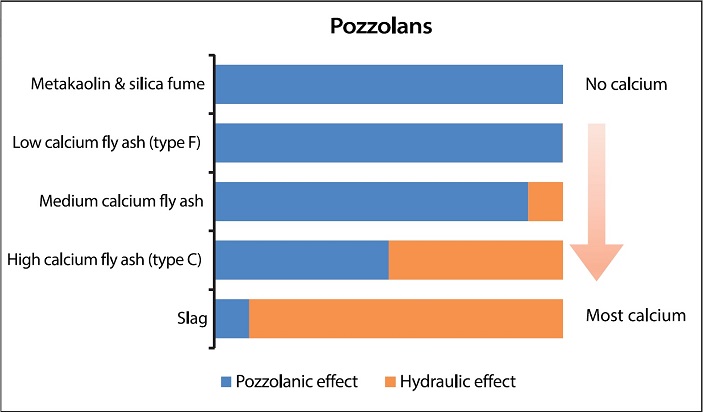 |
| The greater the proportion of calcium oxide contained by a pozzolan, the more it acts as a cementitious material. When added to a mortar, those at the top of the chart act purely as pozzolans, while those at the bottom are inherently hydraulic. Pozzolans with higher hydraulic contents release lime which can block pores and densify a mortar. |
Some pozzolans are not appropriate for use in most conservation. Blast-furnace slag (GGBS/GBFS) is one example of this; it is a limestone which has been calcined at 1500°C along with molten metal, and then cooled and ground. The temperatures and processes are almost exactly like those used to make a Portland cement, and it contains unwanted heavy metals and a high proportion of soluble sulphates. GGBS/GBFS can be used at up to a 95 per cent replacement for Portland cement and still maintains a 32.5N minimum strength (under EN197), the same strength as most CEM IIs on the EU market (these cements are required to contain a minimum of 65% Portland cement).
Of the various limes and natural cements in general use in conservation, there are no inherently bad materials; there is only an appropriateness of specification. A wide range of available materials means we have a vast palette to choose from when deciding how to perform repairs, renovations or restorations. The one downside of this wide spectrum of materials and the complexities of their chemistry is that understanding the properties and interactions of everything on the market becomes nearly impossible. Developing a good specification depends not just on material performance but a collaborative approach between all parties involved.
Sources and recommended reading
English Heritage Practical Building Conservation: Mortars, Renders and Plasters, Ashgate 2011
How Hydraulic Lime Binders Work, Alan M Forster, Scottish Lime Centre 2004 http://bc-url.com/forster-hydraulic
Concrete, Sydney Mindess, Francis Young and David Darwin, Pearson, 2nd edition 2002
Cement and Concrete Research Vol 41, Issue 12, “Supplementary cementitious materials”, Barbara Lothenbach, Karen Scrivener and RD Hooton, Elsevier 2011
Use of Prompt Cement with Natural Hydraulic Limes, Denis Sommain, The Louis Vicat Technical Centre Materials and Microstructures Laboratory, Special Binders Section, 2006
Chemistry and Technology of Lime and Limestone, Robert S Boynton, Wiley, 2nd edition 1980



